veriseq nipt v2
The VeriSeq NIPT Solution v2 assay enables accurate identification of fetal aneuploidy allowing detection of genome-wide fetal chromosomal anomalies with high clinical sensitivities and specificities and a low assay failure rateClinical Trial Notification CTN identification number ID. VeriSeq NIPT Solution v2 is a next-generation sequencing based method to noninvasive prenatal testing Illuminas VeriSeq NIPT Solution v2.

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada.

. You can also use your own pipeline for analysis. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. Instructions for using the VeriSeq NIPT Solution v2.
PDF 1 MB Aug 13 2021. Intuitive Illumina Software Illuminas VeriSeq NIPT Workflow Manager Software includes a graphical interface to guide users through protocol selection and assay setup. Run the RNA-Seq workflow FASTQ only on the MiSeq and stream the data to BaseSpace.
P1 reagents are now available for NextSeq 1000NextSeq 2000 Systems offering added flexibility to meet your projects needs. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. The laboratory can choose to run basic or ge- nome-wide screening by sample.
VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. Aneuploidii plodu pro chromozomy 21 18 13 X a Y lze detekovat s vysokým stupněm přesnosti neinvazivním prenatálním testováním NIPT které využívá celogenomové sekvenování mimo buněčné DNA cfDNA získané z krevní plazmy matky v 10. The BaseSpace RNA-Seq Alignment App analyzes data from the TruSight RNA Pan-Cancer Panel providing a simple results summary that includes a fusion table variant table and gene expression table.
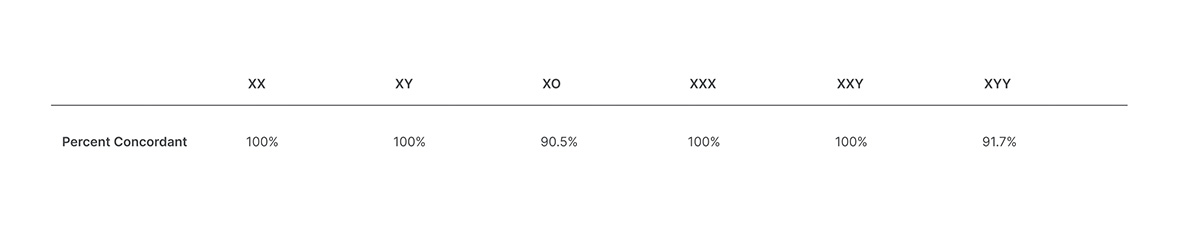
Here archived plasma samples were used. The automated workflow easily scales to analyze 24 48 or 96 samples per run to allow for efficiency and flexibility in managing sample volumes. Concordance of VeriSeqTM v2 for detecting sex chromosome aneuploidies.
Týdnu těhotenství nebo později. Each run including complete sample tracking is summarized in a downloadable report file. Welcome to Immense Discovery Power.
VeriSeq NIPT Solution v2 incorporates automated sample preparation and sequencing data analysis. VeriSeq NIPT Solution v2. CfDNA was extracted from 1 mL of plasma by adsorption onto a binding plate to remove.
Claria NIPT based on Illumina VeriseqTM Solution v2 brings this whole-genome sequencing WGS approach to NIPT. Instructions for processing samples with the VeriSeq NIPT Solution v2. In a clinical setting plasma would be isolated from 710 mL of whole blood from pregnant women 10 weeks gestation.
VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 1 MB. NovaSeq 6000 Reagent Kits v15. The assay provides information about fetal chromosomal status as early as 10.
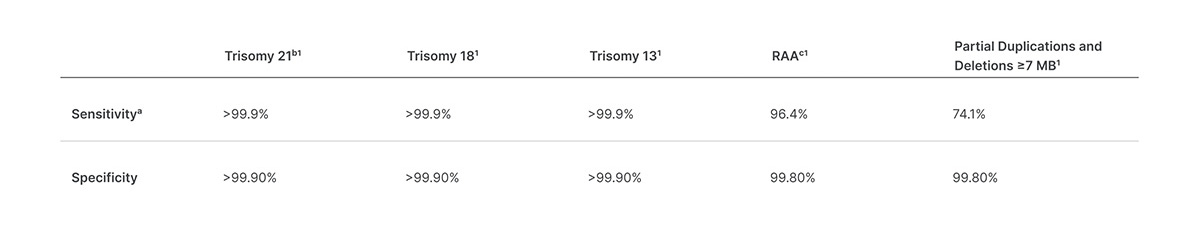
It still offers an automated next-generation sequencing-based workflow that can process up to 96 samples in about a day with PCR-free library preparation. NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs. This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome.
352 28100 549 Monday-Friday 0900-1200 and 1300-1700 Fax. RevisionHistory Document Date DescriptionofChange Document 1000000067940v06 August 2021 UpdatedEUAuthorizedRepresentativeaddress. VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada.
ProductsLearnCompanySupportRecommended Links Products Software Analysis Services Popular Products Instruments Selection Tools. VeriSeq NIPT Solution v2 Consumables Equipment List Consumables and equipment list required for the VeriSeq NIPT Solution v2. Business Wire Illumina has collaborated with Next Generation Genomic NGG Thailand to introduce an automated in-lab IVD solution called VeriSeq NIPT Solution v2 in Thailand.
Like its predecessor the VeriSeq NIPT solution v2 provides information about trisomy 21 13 and 18 as well as some sex chromosome aneuploidy. View Options IVD Symbol Key Symbol key and translations for Illumina IVD products. Unlock a broad range of applications with one powerful instrument.
VeriSeq NIPT Solution Comprehensive and reliable NIPT solution Reagents instruments and CE-IVD marked library prep and analysisreporting software in an automated workflow for in-lab prenatal aneuploidy screening. NextSeq 10002000 Reagents. Percent Concordant XO XXX XXY XYY 9050 100 100 9170.
The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for. Sequencing of the full fetal genome provides a. VeriSeq NIPT Solution v2 Package Insert Translated into.
VeriSeq NIPT Solution v2. NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs. View Options VeriSeq NIPT Solution v2 Software Guide Instructions for use of the software involved with the VeriSeq NIPT Solution v2.
For all queries relating to NIPT please get in touch with the constitutional cytogenetics team at the National Center of Genetics. Welcome to Immense Discovery Power. VeriSeq NIPT v2 - Illumina.
Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. The integrated VeriSeq NIPT Solution v2 provides every - thing needed to run the assay. Improved Q30 score support for UMIs extended shelf life and support for Illumina DNA PCR-Free Library Prep.
VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese.
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Veriseq Pgs Kit Miseq Vitrolife

Illumina Introduces Expanded Version Of Veriseq Nipt Solution Offering More Comprehensive Detection Of Rare Chromosomal Conditions Business Wire

The Veriseq Nipt Solution Youtube

Veriseq Nipt Solution V2 Genetica

Veriseq Nipt Solution V2 Support
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Workflow Manager Guide Pdf Free Download

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Illumina S Noninvasive Prenatal Screening Kit Receives Regulatory Approval In S Korea Business Wire

Comparison Of Aneuploidy Incidence Between Study Cohorts Download Table

Products For In Vitro Diagnostic Use

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Noninvasive Prenatal Testing How Far Can We Reach Detecting Fetal Copy Number Variations European Journal Of Obstetrics And Gynecology And Reproductive Biology

Noninvasive Prenatal Testing How Far Can We Reach Detecting Fetal Copy Number Variations European Journal Of Obstetrics And Gynecology And Reproductive Biology

Illumina Twitter પર Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19
Comments
Post a Comment